R.N. Robertson Travelling Fellowship, Juan De La Cruz Jimenez Serna, UWA.
In my PhD studies at the University of Western Australia, I have focused on the identification of physiological traits associated with the tolerance of the C4 perennial grasses Urochloa spp to waterlogged acidic soils.
In my studies, we have found that root traits including higher aerenchyma percentage, lower stele area, suberized exodermis, lignified sclerenchyma and a barrier to impede the radial O2 loss are helping to improve plant internal aeration, thus, facilitating the O2 movement from shoots to roots and enabling root growth and nutrient uptake under low-O2 conditions. Moreover, from our studies on root radial O2 loss in response to toxic ions (i.e., Fe+2) and low-O2(deoxygenated agar) treatments, we found out that the development of a barrier to impede radial O2 loss also prevents the entry of toxic ions into the roots.
In several meetings with my supervisor Prof. Tim Colmer, we discussed about the results of my research and the need for measuring root respiration and root cellular O2 concentration in plants growing in low-O2 conditions. These measurements are key for understanding the influence of low-O2 conditions on processes such us root growth and nutrient uptake and would provide important data to understand functioning of roots under limited O2 conditions. Fortunately, Prof. Ole Pedersen from the University of Copenhagen visited our lab in Perth in 2017, we discussed with him our data set and he offered the opportunity for me to visit his lab and do the measurements required.
Thanks to the R.N. Robertson Travelling Fellowship, the Australian Society of Plant Scientists and funds from Prof. Tim Colmer and Prof. Ole Pedersen, I was able to visit Prof. Ole’s Lab, the Freshwater Biological Laboratory of the University of Copenhagen in Denmark. Prof. Ole’s lab is a state of the art laboratory with unparalleled facilities for measuring root tissue O2 status. The lab has O2 microelectrodes, micro-manipulators, respiration chambers and the gas mixers needed to run my experiments.
Before travelling to Denmark, I sent seeds from two genotypes (previously evaluated during my PhD) having contrasting root features. The seeds were planted before I arrived and after establishment, the plants were grown in controlled conditions of light and temperature in pots filled with deoxygenated stagnant medium during three weeks (Fig. 1).
Fig. 1. Plants growing in deoxygenated stagnant conditions under controlled light and temperature.
Individual plants were used for measurements of root tissue O2 status by radial profiling across intact roots (from external medium, across the epidermis and outer tissues, cortex and into the stele). The shoots were sealed off in a chamber and the roots were on stirred water at O2 equilibrium (Fig. 2).
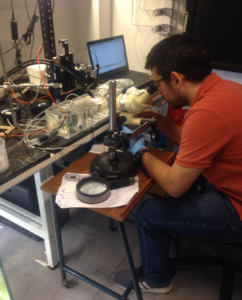
Fig. 2. Experimental set-up. The roots are in stirring water at O2 equilibrium and the shoots are in a chamber with controlled O2 concentration. Microscope is used to position the microelectrode, and O2 profiles are taken by slightly moving the microelectrode using a micromanipulator and a computer system.
The roots were reliant on internal O2 supply via root aerenchyma and the concentration of O2 in shoots was manipulated (21 or 42% O2 concentration) using gas mixers. Moreover, the temperature of the water medium was controlled at 5 or 25 °C.
This research collaboration using an unique set-up has helped us to increase our understanding of tissue O2 status within roots under low-O2 conditions when the external conditions (i.e. O2 concentration and temperature) change. Moreover, in this project we could elucidate some physiological aspects of root aeration under low-O2 conditions. In fact, measurements of O2 profiling have been merely done in wetland plans, thus, this work reinforces the understanding of O2dynamics in C4 grasses. This knowledge adds invaluable significance to my previous findings and adds important data for my PhD thesis.
Fig. 3. From left to right. Prof Ole Pedersen, PhD student Lucas Ogorek, Dr. Elisa Pellegrini and myself.
This project has been very educational for me, having worked side by side with Prof. Ole (an expert and top scientist on responses of plants to low-O2 conditions) and his team (Fig. 3) has contributed to improve my knowledge on O2 transport in plants and my skills in the use of eco-physiology equipment. I am very grateful to the R.N. Robertson Travelling Fellowship, the Australian Society of Plant Scientists, the University of Western Australia, Prof. Tim Colmer and Prof. Ole Pedersen for funding my project. I would also like to thank Lucas, Elisa, Raheb, Ayoe, Ann and many others at the University of Copenhagen that made my stay in Denmark pleasant and productive.
